Atmospheric Water Harvesting (AWH) by Hydrogels for Addressing Global Water Scarcity
Dr. Rohan S. Dassanayake - Senior Lecturer -University of Sri Jayewardenepura
Atmospheric Water Harvesting (AWH) by Hydrogels for Addressing Global Water Scarcity
Summary
Freshwater scarcity is one of the most challenging problems worldwide. However, the Earth’s atmosphere is a ubiquitous water resource which holds approximately 12 900 billion tons of freshwater with rapid replenishment. Atmospheric water harvesting (AWH) technology is a novel approach to address water scarcity by capturing freshwater from ambient air to supply clean water. Among numerous AWH technologies, sorbent-assisted AWH is considered the most sustainable strategy that harvests water from dry air over a broad relative humidity (RH) range in the presence or absence of electricity. Sorbents with water sorption-desorption capabilities play a vital role in this technology. Polymeric hydrogels have been exploited as promising materials for sorbent-assisted AWH due to their intriguing characteristics, including high water uptake, tunable polymeric structure, fast sorption/desorption rates, low cost and energy requirement. Hydrogel-based AWH technology shows the potential to provide a decentralized water supply, especially in regions where water scarcity is immensely impacted.
Although water covers around 70% of earth’s surface, only 3% is freshwater with over two-thirds of that is contained in frozen glaciers, making unavailable for use. Moreover, with the rapid population growth, urbanization and industrialization, environmental pollution and adverse climate changes across the globe have exerted overwhelming pressure on freshwater resources. Hence, the availability of freshwater is shrinking although the demand is rising (Li et al., 2023). According to the United Nations Children’s Emergency Fund (UNICEF), over two billion people reside in countries with inadequate water supply and the half of the world’s population could be living in regions encountering water scarcity by as early as 2025. As a result, providing sufficient freshwater, especially to arid regions and decentralized communities is one of the enormous challenges in the 21st century.
Over the past few years, several water purification technologies have been developed to harvest freshwater from alternative resources, including seawater, rainwater and wastewater (Lu et al., 2023). The most widely applied water purification techniques are ultrafiltration, multistage flash distillation, reverse osmosis, and solar-driven water purification (Zhou et al., 2020). However, these technologies have limitations, including high energy and labor requirements, costly installation, transportation difficulties, inaccessibility to remote, rural and landlocked regions and climate fluctuations, making them unsustainable (Zhou et al., 2020).
Recently, harvesting atmospheric water or moisture in the air has emerged as a novel, sustainable and plausible solution for addressing global freshwater scarcity. Moreover, atmospheric water is ubiquitously available in the air, accounting for approximately 14% of all freshwater in lakes and rivers on Earth (Ahrestani et al., 2023). The total atmospheric moisture is estimated to be 12900 km3 throughout the Earth’s atmosphere, enabling continuous freshwater supply worldwide regardless of the hydrological condition and geographical location (Tashtoush and Alshoubaki, 2023). Several atmospheric water harvesting (AWH) technologies have developed over the last few years.
Atmospheric Water Harvesting (AWH) Technologies
AWH technologies generate water directly from moisture present in the air using easily accessible energy sources such as solar power. Three major AWH technologies are as follows:
- Fog Collection
- Refrigeration
- Sorbent-Assisted Harvesting
Fog collection is the oldest and the most straightforward process, which collects water droplets from moving air using large meshes. The key issues with fog collection are the requirement of high ambient relative humidity (RH) and the sensitivity to climate changes, restricting this process to limited geographical regions (Lu et al., 2023, Li et al., 2018). Refrigeration technology is the broadly employed AWH technology, which utilizes cold surfaces to condense atmospheric air by active cooling below the dew point (Lu et al., 2023). However, this process is energy-intensive as the energy consumption depends on the temperature difference between ambient temperature and dew point, making them impractical to employ in arid regions with low pressures (Gentile et al., 2022). The sorbent-assisted harvesting technology uses various sorbents to adsorb water from the air, followed by heating to release the adsorbed moisture and subsequent condensation to access freshwater. Unlike the other two technologies, sorbent-assisted harvesting is more decentralized and shows tremendous potential in harvesting water over a wide range of RH conditions and geographical regions with a minimal energy requirement (Park et al., 2022). Therefore, sorbent-assisted technology has attracted considerable attention in recent times.
Sorbent-assisted AWH Technology
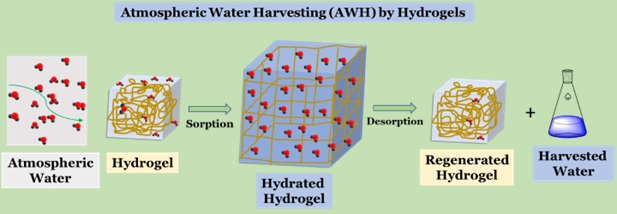
Sorbent-assisted AWH Technology primarily works on the sorption and desorption principle. During the sorption process, the sorbent harvests water from the air. At the desorption stage, the water is released thermally into an enclosed area, generating high RH and enabling condensation (Lu et al., 2023). Therefore, this technology works without a sensible cooling system and high energy demand for vapor condensation (Hanikel et al., 2020). Generally, the efficiency of sorbent-assisted AWH technology relies on the sorption capacity, regeneration temperature and the adsorption/desorption rate of the sorbent (Lu et al., 2023, Park et al., 2022).
Sorbents used in AWH can be mainly divided into liquids and solids. Liquid sorbents are absorbents, while solid sorbents are adsorbents. The absorption process is a bulk phenomenon where the water molecules diffuse into liquid sorbents due to the partial vapor pressure between the air and the surface of the sorbent. On the contrary, the adsorption process is a surface phenomenon where the water molecules attach to the surface of the sorbent. Most widely employed liquid absorbents include the salt solutions of calcium chloride (CaCl2), lithium chloride (LICl), lithium bromide (LiBr) and ionic liquids (Lu et al., 2023). Unlike liquid absorbents, solid adsorbents do not change physically or chemically in the presence of water; hence they have been widely tested for AWH technologies (Lu et al., 2023). Among many sorbents, hydrogels are considered the best candidates for sorbent-assisted water harvesting.
Hydrogel-based Sorbent-assisted AWH
A hydrogel is a hierarchical three-dimensional (3D) cross-linked network of a hydrophilic polymer that can imbibe and retain at least 10% of water by total volume and weight (Dassanayake and Renuka, 2021). The hydrogel products can be categorized according to their source (natural or synthetic), polymeric configuration (amorphous, crystalline, or semi-crystalline), polymeric composition (homopolymeric, copolymeric and multipolymeric), and network electric charge (ionic, nonionic, ampholytic, and zwitterionic).(Ahmed, 2015) Hydrogels inherit unique characteristics, including tunable surface properties, large sorption capacities, high chemical and mechanical stabilities, flexibility and low-cost preparation (Lu et al., 2023). The intrinsic sponge-like micro and nanoporous network structure enable hydrogels to capture water in large amounts through capillary condensation. Due to their thermo-responsive nature, the energy requirement to extract water is less than other AWH technologies. Moreover, hydrogels exhibit fast adsorption/desorption rates, long-term reusability and stability. Following are some recent examples of hydrogel-based sorbent-assisted AWH technologies.
The application of hydrogels in AWH was first demonstrated in 2018 by Yu and Zhao from the Cockrell School of Engineering at the University of Texas at Austin, Austin, Texas, USA, with the fabrication of solar-driven water purification system using a hierarchically nanostructured gel (HNG) prepared from polyvinyl alcohol (PVA) and polypyrrole (PPy) that harvests water from any source (Zhao et al., 2018). Zhao and coworkers displayed a daily freshwater yield of 18–23 Lm−2 under natural sunlight (Zhao et al., 2018). Li et al. devised a flexible photothermal hybrid hydrogel using polyacrylamide with CaCl2 salt calcium chloride and carbon nanotubes for AWH (Li et al., 2018). The authors reported freshwater uptakes of 0.74, 1.10, and 1.75 g for one gram of the dry hybrid hydrogel (gg−1) under RH of 35, 60 and 80%, respectively, and release of approximately 100% of the captured water under irradiation with regular sunlight (Li et al., 2018). Graeber et al. synthesized a high LiCl salt-loaded polyacrylamide (PAM) superabsorbent hydrogel and demonstrated superior water capturing over a range of RH (Graeber et al., 2023). This leakage-free salt-loaded superabsorbent hydrogel exhibited water uptake capacities of 1.79, 2.58, and 3.86 gg−1 at an RH of 30%, 50%, and 70%, respectively, exceeding water uptake capacities of MOFs by over 100% reported previously (Graeber et al., 2023). The authors mentioned that this superabsorbent hydrogel opens up possibilities for passive water harvesting, especially in arid and drought-prone regions (Graeber et al., 2023). Solar-driven CaCl2 salt-loaded nanostructured hybrid hydrogel prepared from polyacrylonitrile (PAN), acrylamide monomer (AM), multi-walled carbon nanotubes (MWCNTs), and graphene has also been investigated for water harvesting from atmospheric air (Uddin et al., 2022). This hybrid hydrogel (PAN/AM/graphene/CaCl2) showed a water uptake of over one litre (L) per kilogram of the hydrogel (Lkg-1) at an RH of 60% (Uddin et al., 2022). Biopolymer-based hydrogels have also been investigated for sorbent-assisted AWH (Li et al., 2023). For instance, Gao et al. prepared a biopolymer-based hydrogel using glucomannan (KGM) and hydroxypropyl cellulose (HPC) with LiCl. They demonstrated water uptakes of 5.8 and 13.3 Lkg-1 at 15% and 30% RH in arid conditions (Guo et al., 2022). Furthermore, Zhang and coworkers developed a starch-derived photo-responsive AWH sorbent with an acrylic acid (AA)/acrylamide (AM)-grafted starch hydrogel as the backbone, carboxylated multi-walled CNTs as the solar absorber, and LiCl as the water absorber (Zhang et al., 2023). The prepared hydrogel showed a maximum water adsorption capacity of 8.5 gg−1 at an RH of 90% (Zhang et al., 2023).
In conclusion, numerous studies conducted on AWH by different types of hydrogels have indicated outstanding water sorption capacities with scalability, stability, versatility, recyclability, less energy, labor and installation cost. Interestingly, biopolymer-based hydrogels are also promising, with increasing emphasis on sustainability, renewability, nontoxicity, and biocompatibility of AWH sorbents. In the future, hydrogels-based sorbent-assisted AWH technology show the potential to address global water scarcity and provide a cost-effective, energy-efficient and decentralized water supply, especially to off-grid, remote, rural, arid, and landlocked areas worldwide.
References
Ahmed, E. M. 2015 ‘Hydrogel: Preparation, characterization, and applications: A review’. Journal of Advanced Research, 6 (2), 105-121.
Ahrestani, Z., Sadeghzadeh, S. & Motejadded Emrooz, H. B. 2023 ‘An overview of atmospheric water harvesting methods, the inevitable path of the future in water supply’. RSC Advances, 13 (15), 10273-10307.
Dassanayake, R. S. & Renuka, N. 2021 ‘Hydrogels: Next Generation Atmospheric Water Harvesting Materials’. Advances in Technology, 1 (1), 1-4.
Gentile, V., Bozlar, M., Meggers, F. & Simonetti, M. 2022 ‘Liter-scale atmospheric water harvesting for dry climates driven by low temperature solar heat’. Energy, 254, 124295.
Graeber, G., Díaz-Marín, C. D., Gaugler, L. C., Zhong, Y., El Fil, B., Liu, X. & Wang, E. N. 2023 ‘Extreme Water Uptake of Hygroscopic Hydrogels through Maximized Swelling-Induced Salt Loading’. Advanced Materials, 2211783.
Guo, Y., Guan, W., Lei, C., Lu, H., Shi, W. & Yu, G. 2022 ‘Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid environments’. Nature Communications, 13 (1), 2761-2768.
Hanikel, N., Prévot, M. S. & Yaghi, O. M. 2020 ‘MOF water harvesters’. Nature Nanotechnology, 15, 348-355.
Li, R., Shi, Y., Alsaedi, M., Wu, M., Shi, L. & Wang, P. 2018 ‘Hybrid Hydrogel with High Water Vapor Harvesting Capacity for Deployable Solar-Driven Atmospheric Water Generator’. Environmental Science & Technology, 52, 11367-11377.
Li, S., Hernandez, S. & Salazar, N. 2023 ‘Biopolymer-Based Hydrogels for Harvesting Water from Humid Air: A Review’. Sustainability, 15, 848.
Lu, W., Ong, W. L. & Ho, G. W. 2023 ‘Advances in harvesting water and energy from ubiquitous atmospheric moisture’. Journal of Materials Chemistry A, 11 (24), 12456-12481.
Park, H., Haechler, I., Schnoering, G., Ponte, M. D., Schutzius, T. M. & Poulikakos, D. 2022 ‘Enhanced Atmospheric Water Harvesting with Sunlight-Activated Sorption Ratcheting’. ACS Applied Materials & Interfaces, 14 (1), 2237-2245.
Tashtoush, B. & Alshoubaki, A. 2023 ‘Atmospheric water harvesting: A review of techniques, performance, renewable energy solutions, and feasibility’. Energy, 280, 128186.
Uddin, M. N., Rab, M. F., Islam, A. K. M. N., Asmatulu, E., Rahman, M. M. & Asmatulu, R. 2022 ‘Nanostructured Hybrid Hydrogels for Solar-Driven Clean Water Harvesting from the Atmosphere’. Materials, 15 (21), 7538.
Zhang, H., Zhou, Z., Du, J., Pei, X. & Zhou, L. 2023 ‘Starch-derived photoresponsive high-efficientcy hygroscopic hydrogel for all-weather atmospheric water harvesting’. Journal of Cleaner Production, 416, 137897.
Zhao, F., Zhou, X., Shi, Y., Qian, X., Alexander, M., Zhao, X., Mendez, S., Yang, R., Qu, L. & Yu, G. 2018 ‘Highly efficient solar vapour generation via hierarchically nanostructured gels’. Nature Nanotechnology, 13 (6), 489-495.
Zhou, X., Lu, H., Zhao, F. & Yu, G. 2020 ‘Atmospheric Water Harvesting: A Review of Material and Structural Designs’. ACS Materials Letters, 2 (7), 671-684.

Dr. Rohan S. Dassanayake
Senior Lecturer
Department of Biosystems Technology, Faculty of Technology
University of Sri Jayewardenepura

