Hydrogen for the Future World: Current Production Techniques for a Green Transition
Dr. S. P Ratnayake

We have been powering our world through fossil fuel for ages and even in this space-age, around 80% of our energy still comes from fossil fuels [1]. Fossil fuel burning has environmentally detrimental consequences and produces greenhouse gases which cause global warming and climate change which adversely affect human wellbeing.
As of today, close to 80% of energy needs are fulfilled via burning fossil fuels while around 18% and 2% are secured from renewable energy and nuclear energy, respectively [1]. If the current renewable energy quota of 18% is separated to its components, it is observed that around 7% of it comes from traditional biomass which is not entirely environment friendly [1]. The actual percentage of truly green energy within the global energy supply is surprisingly minute.
Hydrogen has emerged as a clean, alternative energy source with the potential to substitute currently widespread fossil fuel-based energy forms. Production and utilization of hydrogen for industrial and automotive uses has been there for a while yet almost all the hydrogen generated into the modern world come from either coal gasification or methane steam reformation which are directly linked to fossil fuels [2]. Within the current renewable energy picture there is no place for hydrogen due to this factor.
Given the upsurging public interest and market capitalization on hydrogen, techniques which can be utilised to generate hydrogen without the involvement of fossil fuels has been a topic of widespread scientific interest lately. These methods can actually be called as green or truly renewable hydrogen routes and hold utmost importance concerning the current global drive to curb the utilization and dependence on fossil fuels. Among techniques of green hydrogen generation, electrolysis of water and solar-driven water splitting have shown promise.
Electrolysis of water involves decomposition of water via driving an electric current through water. This is achieved via immersion of two platinum electrodes in water added with an electrolyte to make water a conducting medium. As the current flows through the system, water molecules are oxidised at the anode producing hydrogen while water molecules are reduced at the cathode giving rise to oxygen. This reaction is an uphill reaction and needs a Gibbs free energy of 237 kJ/mol [3]. The individual reactions taking place in a neutral solution at each electrode and the overall reaction are denoted below (Fig 1)[4].
2H2O O2 + 4H+ + 4e– (Anode)
2H2O + 2e– H2 + 2OH– (Cathode)
2H2O O2 + 2H2 (Overall reaction)
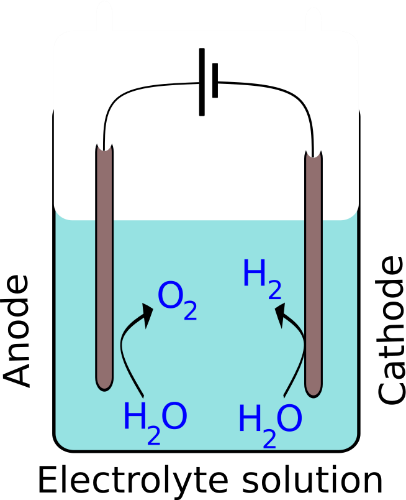
One of the green approaches to generate hydrogen via electrolysis of water is to drive this reaction by electricity generated through photovoltaic cells. However, the power generated by standard photovoltaic cells are limited, offsetting the convenience of such systems. Yet, current solar photovoltaics are stable and have the capability to power these simple electrolytic cells without the hazzle of specialised maintenance.
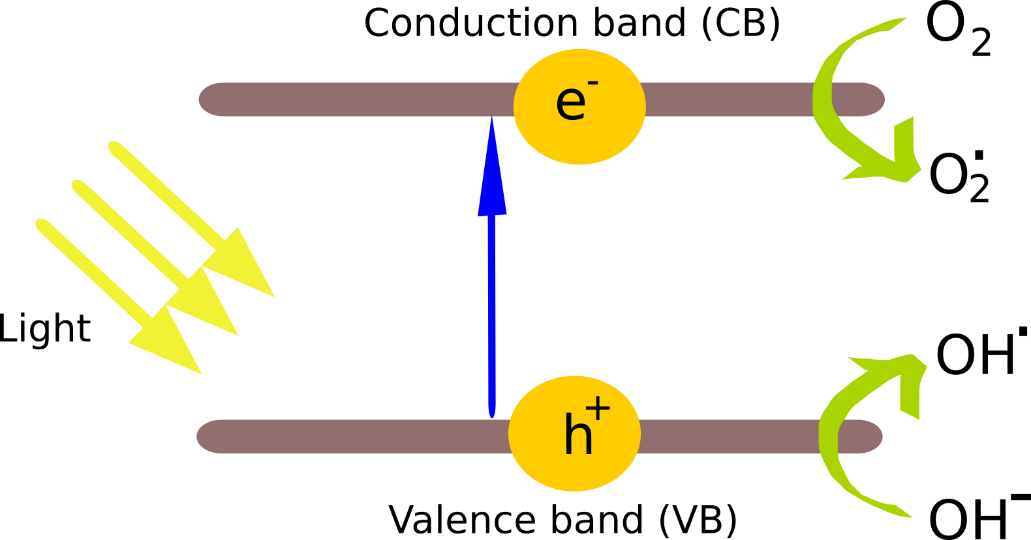
In addition to this approach, these has been growing interest on another technique which is known as the photocatalytic water splitting. In this method, the absorption of solar radiation and the splitting of water is conducted at a single site [5]. The advantage of this system is that since the solar radiation is utilised to catalyse the water splitting reaction, the activation energy barrier of the reaction is reduced. This results in a reduction of the amount of energy that has to be supplied for the water splitting reaction to proceed. The process is dependent upon the band gap of the semiconducting material employed as the radiation absorbing substrate and this band gap must straddle the oxidation (1.23 V) and reduction (0 V) redox potentials of water for the successful progressing of the reaction [6]. Basis of this process is the traditional photocatalytic phenomena discovered by Honda and Fujishima in 1972 [7]. The basic photocatalytic mechanism is depicted below (Fig 2).
When the semiconductor is exposed to radiation with sufficient photon energy to excite electrons from the valence band to the conduction band, a charge separation takes place leaving an electron vacancy/hole in the valence band and an electron in the conduction band. Eventually, if the recombination of the two does not occur and the semiconductor is in the vicinity of hydroxyl ions (OH–) and oxygen, holes are consumed by OH– while electrons are acquired by O2 to form free radicals: OH* and O2*, respectively. The charge separation is the key aspect of the photocatalytic process and formed reactive oxygen species (ROS) and OH* free radicles can be incorporated into numerous ubiquitous applications such as disinfection and degradation of organic contaminants.
As the water-splitting process involves a Gibbs free energy of 237 kJ/mol, in a photoelectrochemical (PEC) system, this energy is to be provided by photons incident upon the photoanode. The propagation of both H2 and O2 generation reactions in the same vicinity is unfavourable due to charge recombination. For an efficient water splitting process, electrons are transferred via an external circuit to a different reaction site termed the counter electrode, whereas generated holes undergo reaction at the illumination site which becomes the working electrode (photoanode). In this case as well, the electrolyte solution must possess a decent conductivity for the overall reaction to take place. The scheme of a photoelectrochemical water splitting system is depicted below (Fig 3).
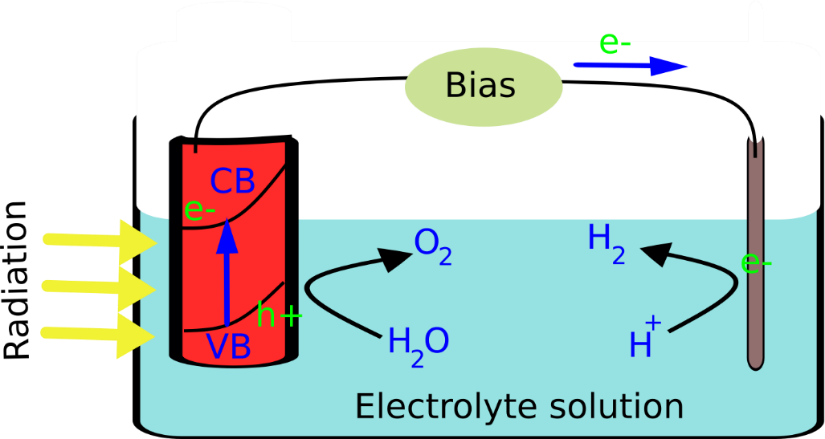
As described above, the photoinduced charge separation is a phenomenon that has profound implications. PEC water splitting is only an example of how this action can be converted to useful means.
To pave the way towards a world powered by green energy, transition from currently abundant fossil fuel towards environmentally benign hydrogen-type fuels is instrumental. As of today, the technology behind these green hydrogen techniques is still primitive, hence an immediate transition out of fossil fuels into these sources are unlikely. However, numerous researchers are currently working on routes which can be utilised to improve the efficiencies of these green hydrogen generation techniques and devices which can function under realistic environmental conditions are slowly emerging. The main venue which requires improvement is the absorbance and conversion efficiency of solar cells and photoactive semiconductor materials. With current technology on solar photovoltaics and other types of semiconducting materials advancing at a significant rate, the introduction of commercial scale solar-driven hydrogen generation can be anticipated in the near future.
References
- REN21_Secretariat-Paris, Renewables 2020 Global Status Report. 2020(https://www.ren21.net/reports/global-status-report/): p. 32.
- T-raissi, A. and D.L. Block, Hydrogen: automotive fuel of the future. IEEE Power and Energy Magazine, 2004. 2(6): p. 40-45.
- Rajaambal, S., K. Sivaranjani, and C.S. Gopinath, Recent developments in solar H2 generation from water splitting. Journal of Chemical Sciences, 2015. 127(1): p. 33-47.
- Cheng, Y., Advances in electrocatalysts for oxygen evolution reaction of water electrolysis-from metal oxides to carbon nanotubes. Progress in natural science: materials international, 2015. 25(6): p. 545-553.
- Maeda, K. and K. Domen, Photocatalytic water splitting: recent progress and future challenges. The Journal of Physical Chemistry Letters, 2010. 1(18): p. 2655-2661.
- Cui, Y., et al., Two-dimensional few-layer group-III metal monochalcogenides as effective photocatalysts for overall water splitting in the visible range. Journal of Materials Chemistry A, 2018. 6(45): p. 22768-22777.
- Fujishima, A. and K. Honda, Electrochemical photolysis of water at a semiconductor electrode. nature, 1972. 238(5358): p. 37-38.


